|
|
 |
History and introduction.
- In 1882, Langenbuch performed one of the first cholecystectomies.1 He was later quoted as saying "the gallbladder should be removed not because it contains stones, but because it forms stones."2 Surgical removal of the gallbladder thus became the gold standard for management of biliary calculus disease.
- Credit for performing the first procedure is now given to Dr. Erich Muhe of Germany.3 In September 1985 he performed his first laparoscopic cholecystectomy, but his efforts were lost to the world.
- In 1987, the French surgeon Philippe Mouret performed his first laparoscopic cholecystectomy while performing laparoscopy on one of his gynecology patients.
- In May 1988, Dr. Dubois performed his first laparoscopic cholecystectomy, 4 and after presenting his work to his colleagues, awoke interest in France.
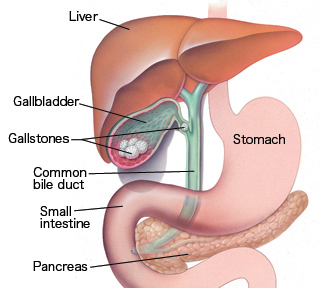
Surgical Anatomy of the Gallbladder and Biliary Tree.
Discussion of laparoscopic cholecystectomy is incomplete without a review of the anatomy of the gall bladder and the biliary tree.
Common Hepatic Duct
- The common hepatic duct is the length of biliary duct from the hepatic duct confluence to the cystic duct. The common hepatic duct makes up the left border of the tri- angle of Calot. The length of common hepatic duct varies from 1 to l0cm depending on the location of the junction with the cystic duct.5 where it then becomes the common bile duct.'
- The common hepatic duct can often be associated with accessory ducts. They are readily injured at cholecystectomy if they traverse the tri- angle of Calot. In more than half of the cases in which an accessory duct is found, it joins the common hepatic duct somewhere along its course. Less frequently, the accessory duct joins the cystic duct. In the rarest of instances. It may join a duct in the opposite lobe. The majority of aberrant ducts are on the right side.
Gallbladder
- The gallbladder is a pear-shaped, distensible appendage of the extrahepatic biliary system, usually holding 30 to 50 ml of bile. The gall bladder is attached to the liver by areolar connective tissue that contains multiple small lymphatics and veins.
- Rarely, one or more small accessory bile ducts pass through this tissue to enter the gallbladder directly (ducts of Luschka).6 In extremely unusual cases, major hepatic ducts might even drain directly into the gallbladder.
- This intimate relationship to the visceral surface of the liver easily permits direct spread of gallbladder inflammation, infection or neoplasia into the parenchyma of the liver. The transition between the neck and the cystic duct can be gradual or abrupt. The neck is quite short, usually 5 to 7mm.7
- Unusual morphologies of the gallbladder including septations or duplications or even agenesis may occasionally present during laparotomy or laparoscopy. A septated gallbladder is by definition a bilobar gallbladder with a single cystic duct but two fundi. Duplication of the gallbladder means the presence of two cystic ducts. A double cystic duct draining a unilocular gallbladder has once been described. More frequently encountered anomalies of the cystic duct and gallbladder are intrahepatic gallbladders and a gallbladder within the left lobe of the liver. 16
Cystic Duct
- The cystic duct is the route by which the gallbladder empties its bile. It connects the neck of the gallbladder to the common hepatic duct. In as many as 10% of cases, a portion of the right hepatic biliary system joins the cystic duct before its junction with the common hepatic duct. Generally, the cystic duct is about 4cm long. The length may vary from 0.5 to 8cm depending on the site of the gallbladder and the junction with the common hepatic duct. The circumference of the duct varies from 3 to 12mm.8
- As a general rule, the cystic duct joins the right aspect of the common hepatic duct. The cystic duct may (1) join the common hepatic duct at various angles; (2) be parallel to the right side of the common hepatic duct before entering it; (3) be dorsal to the common duct and enter its dorsal surface; (4) be dorsal to the common duct and enter it from the left side; (5) enter the right or left hepatic duct directly; or (6) join the common duct just before it enters the posteromedial wall of the duodenum. The mode of entrance of the cystic duct into the common hepatic duct may be angular, parallel, or spiral. The variable site of the union of the hepatic and cystic ducts determines the length of the common bile duct. If this union is low, that is, distal within the porta hepatis near the duodenum, the supraduodenal portion of the common bile duct is very short or even absent. If this is the case, the cystic and common hepatic ducts run parallel for a considerable length, causing difficulties during cholecystectomy. The cystic duct may also be very short or absent, in which case the gallbladder may appear to empty directly into the common hepatic duct.
|
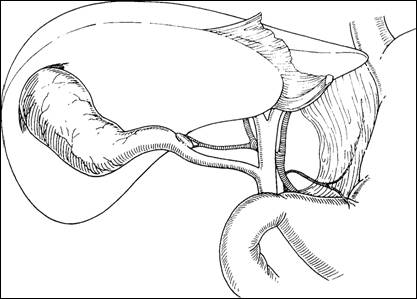
Triangle of Calot and Rouviere's Sulcus
- Calots triangle is the region bounded by the cystic duct, common (or right) hepatic duct, and inferior border of the liver. Bile duct injuries during cholecystectomy most frequently occur because of poor exposure of Calot's triangle, leading to confusion between the common hepatic or common bile duct and the cystic duct.
- The apex of the triangle contains the cystic artery, as well as the right branch of the hepatic artery, 95% of accessory right hepatic arteries, and 90% of accessory bile ducts. An anomalous hepatic artery arising from the superior mesenteric trunk (replaced right hepatic artery) usually courses superiorly in the groove posterolateral to the common bile duct. Therefore, it appears on the medial side of the apex of Calot's triangle, just behind the cystic duct where it is vulnerable to injury during cholecystectomy.
- Another landmark in this region that can be helpful in identifying the plane of the common bile duct and avoiding injuries during cholecystectomy is Rouviere's sulcus, identified by Rouviere in 1924 as a 2- to 5-cm sulcus lying anterior to the caudate lobe and running to the right of the liver hilum and usually containing the right portal triad.
Common bile duct
- The junction of the common hepatic duct with the cystic duct forms the common bile duct. The length of the common bile duct is variable, reported in adults as short as 1cm and as long as 17 cm, After cholecystectomy, the normal common bile duct may dilate to 10 to 12mm.
- Once the common bile duct has formed by the junction of the cystic and common hepatic ducts, it is designated as the supraduodenal segment of the common bile duct. Subsequently, it becomes the retroduodenal portion that in turn leads to the pancreatic and eventually the intraduodenal segments of the common bile duct.
Arterial Supply
- The hepatic artery supplies approximately 25% of the total blood flow to the liver; however, it provides up to 75% of the oxygenated blood and about 85% to 90% of the blood to the extrahepatic biliary system.
- Because of abundant collaterals, ligation of the hepatic artery proximal to the gastroduodenal artery fails to damage the liver.
- Ligation of the hepatic artery distal to the gastroduodenal artery occasionally produces hepatic necrosis Usually, however, this does not result in serious consequences because there are also rich extrinsic collaterals to the hepatic artery beyond the gastroduodenal artery. Ligation of the right or left hepatic artery individually predictably results in marked elevation of hepatic enzyme levels but often still without severe clinical manifestations,
- The gallbladder receives its blood supply from the cystic artery. The cystic artery usually originates from the right hepatic artery shortly after it passes beneath the common hepatic duct.
- The site of origin of the cystic artery varies greatly, however. The more common variations are from an aberrant right hepatic artery, left hepatic artery, more proximal hepatic artery, gastroduodenal; or even another branch of the celiac artery. In about 10% of cases, a double cystic artery is present. In most cases, the cystic artery branches near the neck of the gallbladder.
- The blood supply of the common bile duct classically arises from the cystic artery or the posterior superior- pancreaticoduodenal artery.
Venous drainage
- There is no constant single venous trunk of the gall bladder. Venous return from the gall bladder occurs in multiple directions, via multiple small vessels running directly into the liver bed or towards the common duct.
- The ventral surface of the common duct is marked by a constant ascending vein that can become a hindrance if bleeding from this vessel cannot be controlled during duct surgery.
Lymphatics
- The lymphatic drainage of the gallbladder is into cystic duct nodes near the superior aspect of the cystic duct or directly into the hepatic parenchyma.
- Numerous lymphatics traverse the connective tissue between the gall- bladder and its bed in the liver. This lymphatic (and adjacent venous) drainage accounts for the high rate of local invasion seen with gallbladder malignancies.
- The lymphatic drainage from the common bile duct courses superiorly and inferiorly into nodes that lie along the course of the duct and finally into a group of 6 to 10 nodes in the porta hepatis. Some lymphatic drainage from the common duct reaches the deep pancreatic group of nodes, situated near the origin of the superior mesenteric artery, but usually the drainage reaches into the deep celiac nodal group.
Anatomic Changes from Gallbladder and Biliary Pathology
- Cholecystitis, as the name suggests, is marked by acute' and/or chronic forms of inflammation of the gallbladder wall. Both acute and chronic cholecystitis are notable for significant anatomic changes seen at the time of cholecystectomy. The most significant of these findings is the abundance of adhesions surrounding the gallbladder. Retraction difficulty is seen in empyema with a gallbladder containing pus or in hydrops when the gallbladder distends with mucoid material secondary to outlet obstruction.
- Mirizzi syndrome is described as jaundice caused by an impacted stone in the gallbladder neck or cystic duct leading to external compression and obstruction of the common hepatic duct. This definition was expanded to two types in the 1980s. Type 1 is characterized by common hepatic duct obstruction by external compression (stone, tumor, lymphadenopathy, etc.) whereas type II is obstruction due to stone passage through a cholecystocholedochal fistula resulting from pressure necrosis between the gallbladder or cystic duct and common hepatic duct. Both are very rare, occurring in 0.7% to 1.4% of all cholecystectomies performed, but can have a high occurrence of gallbladder carcinoma (up to 28% of cases ).9 The nature of the condition in both types requires very close proximity of the gallbladder or cystic duct to the common hepatic duct. .This proximity, in combination with the significant inflammatory changes in the triangle of Calot intrinsic to the syndrome, makes anatomic differentiation of the ducts difficult during surgical dissection.10
- Pancreatitis is also known to create anatomic changes affecting the ability to perform laparoscopic cholecystectomy. The intense retroperitoneal inflammation and edema that can accompany pancreatitis can have a mass effect on adjacent structures, leading to widening of the duodenal C loop, anterior displacement of the stomach, and duodenal mucosal thickening. These changes in addition to possible intraperitoneal inflammation or fluid collections can make adequate exposure of the gallbladder fossa and Calor's triangle difficult.11
- Cirrhosis and its anatomic changes may not directly affect the gallbladder but can make the surgical approach difficult. Associated portal hypertension can lead to the formation of varices leading to difficulty with exposure. Among these varices is the umbilical vein, which is open to create collaterals from the left portal vein to the epigastric vessels (caput medusa), and therefore presents a direct obstruction between the umbilical trocar site and the gallbladder during laparoscopic cholecystectomy.12
- Other less common pathophysiological changes of the gallbladder can cause difficulty during cholecystectomy as well. Examples of these conditions include gallbladder diverticula and adenomyomatosis of the gallbladder. Diverticular disease of the gallbladder, similar to that of the colon, includes true and false diverticula. This complication can lead to trouble during resection caused by chronic scarring of the diverticula to surrounding structures or even intrahepatic diverticula, necessitating a subtotal cholecystectomy to avoid significant hepatic injury or bleeding. 13 Adenomyomatosis also leads to similar changes of scarring or intrahepatic extensions, making cholecystectomy challenging. It is an acquired disease characterized by localized or diffuse extensions of gallbladder mucosa into, and often beyond, the muscular layer of the wall.
Investigations
- Apart from routine blood tests, LFT, PT are must.
- Ultrasonography
- CECT / MRCP in case picture is not clear with ultra sound .
- HIDA SCAN or Cholescintigraphy
A cholescintigraphy or Hepatobiliary Imino-Diacetic Acid scan, (HIDA scan) is a nuclear imaging procedure to evaluate the health and function of the gallbladder. A radioactive tracer, usually technetium-99m, is injected through any accessible vein, then allowed to circulate to the liver, where it is excreted into the biliary system and stored by the gallbladder and biliary system.[1]
Operative Technique
- Laparoscopic cholecystectomy:
- The first step in laparoscopic cholecystectomy is the creation of pneumoperitoneum and the insertion of an initial trocar through which the laparoscope can be passed.
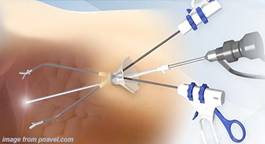
- In most cases, four ports are necessary. The first port is for the laparoscope; the remaining ports are for grasping forceps, dissectors, and clip applier. The precise position of the accessory ports depends on the surgeon's preference, the patient's body habitus, and the presence or absence of previous scars or intra-abdominal adhesions. In some cases, a fifth trocar is required to elevate a floppy liver or to depress or retract the omentum or a bulky hepatic flexure of the colon .
- Obtaining adequate exposure of Calot's triangle is a key step. First, the patient is placed in a reverse Trendelenburg position, with the table rotated toward the left side. Next, the fundus of the gallbladder and the right lobe of the liver are elevated toward the patient's right shoulder.
- One grasping forceps, inserted through the most lateral right-side port and held by an assistant, is placed on the fundus of the gallbladder and the gallbladder is retracted superiorly and laterally above the right hepatic lobe.
- In some patients, such as those with acute cholecystitis and hydrops of the gallbladder, the gallbladder is tense and distended, making it difficult to grasp and easy to tear. In these patients, retraction of the fundus is difficult, and exposure of Calot's triangle is unsatisfactory. This problem is best managed by aspirating the contents of the gallbladder either percutaneously with a 14- or 16-gauge needle inserted into the fundus of the gallbladder under laparoscopic vision or by using the 5 mm trocar in the right upper abdomen to puncture the fundus and then aspirate with the suction irrigator. The surgeon maneuvers Hartmann's pouch to provide various angles for safe dissection of Calot's triangle. Initially, lateral and inferior traction are placed on Hartmann's pouch, opening up the angle between the cystic duct and the common ducts.
- A large stone impacted in the gallbladder neck may impede the surgeon’s ability to place the forceps on Hartmann's pouch, This problem can usually be managed by dislodging the stone early in the operation, as follow: the gallbladder is grasped as low as possible with one grasping forceps; a widely opening dissecting instrument, such as a right-angle dissector, a Babcock forceps, or a curved dissector, is used to dislodge the stone and milk it up toward the fundus; with the same forceps or another large grasper, the stone is held up and away from the neck of the gallbladder, and appropriate retraction is provided.(l7)
- In some problem cases, edema, fibrosis, and adhesions make identification of the gallbladder cystic duct junction very difficult. An anatomic landmark on the liver known as Rouvier's sulcus may be helpful in such circumstance. This sulcus, or the remnant of it, is present in 70% to 80% of live" and usually contain, the right portal triad or its branches. Its location is consistently to the right of the hepatic hilum and anterior to the caudate process (Couinaud segment 1). 'This landmark reliably indicate the plane of the CBD.
- Dissection should always take place at the gallbladder-cystic duct junction, staying close to the gallbladder to avoid inadvertent injury to the CBD. cystic duct lymph node is a useful landmark at this location and may facilitate identification of the gallbladder cystic duct junction.
- Dissection of Calot's triangle should be completed before the cystic duct is clipped or divided. The cystic duct should be dissected for a length sufficient to permit secure placement of two clips; it is not necessary, and indeed may be hazardous, to attempt to dissect the cystic duct-CBD junction.
- The cystic artery is exposed next. A small vein can usually be identified in the space between the cystic duct and the cystic artery; it can usually be pulled up anteriorly and cauterized.
- At this point, the cystic duct is clipped on the gallbladder side three or four clips should be placed on the cystic duct and the cystic duct divided between them. Two or three hemostatic clips are placed on the cystic artery, and the vessel is divided. Delayed injuries to the CBD may be caused by a direct bum to the duct or by sparking from non insulated instruments or clips during dissection.(l5, 16) An alternative is to use locking polymer clips that fit through 5 mm ports, clip across a greater width of tissue, and do not conduct electricity.
- A two-handed approach by the surgeon facilitates this dissection. It is sometimes helpful to apply downward and lateral traction on the forceps grasping the fundus. Dissection continues until the gallbladder is attached only by a small piece of peritoneum at the fundus. Before the last attachment to the gallbladder is completely divided, the vital clips are re inspected to ensure that they have not slipped off, and the operative field is checked for hemostasis and the presence of any bile leakage.
- The final attachment to the gallbladder is then divided. The gallbladder is placed over the right lobe of the liver and laterally so that it can be found again to be retrieved. The grasping forceps on the gallbladder should not be removed.
- The gallbladder may be accidentally breached at some point in the operation, with the result that bile and stones spill into the peritoneal cavity efforts should be made to suction the spilled bile, which accumulates in the suprahepatic space, the right subhepatic space, and the lower abdomen because of the patient's position. Each of these areas should be irrigated and the effluent aspirated until it is clear. Stones should be located and removed whenever possible. (18,19)
- If the gallbladder is perforated and it seems likely that multiple stones will be spilled, the surgeon should introduce a sterile bag into the peritoneal cavity, placing it close to the perforation. Spilled stones can then be transferred immediately into the bag. After the gallbladder is removed from the liver bed, it too is placed in the bag, affording some protection to the wound when it is removed from the abdominal cavity.
- The laparoscope is moved to the epigastric port, and a large-tooth grasping forceps is inserted through the umbilical port to grasp the gallbladder at the area of the cystic duct. Under direct vision, the gallbladder is then retrieved and pulled out as far as possible through the umbilical port. If the gallbladder is small enough, it can be drawn right into the trocar sleeve, and it and the trocar can then be removed together. It is sometimes necessary to stretch the fascial opening with a Kelly clamp or to aspirate bile from the gallbladder. It is far preferable to enlarge the incision than to have stones or bile spill into the abdominal cavity from a ripped gallbladder.
- The fascial opening at the umbilicus should be sutured closed to prevent , subsequent herniation, and all skin incisions should be c1osed.
- The decision to place a drain after laparoscopic cholecystectomy should be governed by the same principles applied to patient" undergoing open cholecystectomy. There are two main indication, for drainage; (1) the cystic duct was not closed securely, and (2) the CBD was explored by either a direct or a transcystic approach. Drain placement is easily accomplished. A closed suction drain is inserted intra-abdominally through the 10 mm operative port. A grasping forceps placed through the right lateral port is used to pull one end of the drain out through the abdominal wall. The other end is then positioned according to the surgeon's preference, usually in the subhepatic space.
- Dissection of gall bladder
- Ligation of cystic duct
- Proximal clipping of cystic duct
1. dissection of gall bladder from fossa
2. final removal of gall bladder
3. insertion of gall bladder in sterile plastic bag
1. Gall bladder with stones
2. Gall stones
3. Gall stones
- CBD exploration becomes essential when it is dilated or there is dilatation of intra hepatic biliary radicles i.e. there is obstruction to the flow of bile through the common duct due to stones in the CBD or stricture of CBD or neoplasm of the biliary tree.
- About 10% of all patients undergoing cholecystectomy for symptomatic gallstones will also have choledocholithiasis. Patients with obvious clinical jaundice or cholangitis, a dilated CBD, or stones visualized in the CBD on preoperative ultrasonography are likely to have choledocholithiasis (risk > 50%).
- Patients who have a history of jaundice or pancreatitis, moderately elevated preoperative levels of alkaline phosphatase or bilirubin, or ultrasonographic evidence of multiple small gallstones are somewhat less likely to have choledocholithiasis (risk, 10% to 50%).
- Patients with large gallstones, no history of jaundice or pancreatitis, and normal liver function are unlikely to have choledocholithiasis (risk < 5%).
- Many methods are available for the exploration of CBD like open choledocholithotomy, ERCP (endoscopic retrograde cholangiole pancreatography), laparoscopic CBD exploration.
- The cholangiogram is reviewed; the size of the cystic duct, the site where the cystic duct inserts into the CBD, and the size and location of the CBD stones all contribute to the success or failure of transcystic CBD exploration. For example, transcystic exploration is extremely challenging in a patient who has a long, spiraling cystic duct with a medial insertion.
- The size of the stones to be removed dictates the· approach to the CBD: stones smaller that 4mm can usually be retrieved in fluoroscopically directed baskets and generally do not necessitate cystic duct dilatation; larger stones (4 to 8mm) are retrieved under direct vision with the choledochoscope.
- A hydrophilic guide wire is inserted through the cholangiogram catheter into the CBD under fluoroscopic guidance. . The cholangiogram catheter is then removed. If the largest stones are larger that the cystic duct, dilatation of the duct is necessary, not only for passage of the stones but also to allow passage of the choledochoscope, which may be 3 to 5 mm in diameter.
- Dilatation is accomplished with either a balloon dilator or sequential plastic dilators. The cystic duct should not be dilated to a diameter greater than 8 mm. Larger stones in the CBD may be either fragmented with electrohydraulic or mechanical lithotripsy, if available, or removed via choledochotomy.
- Once dilatation is complete, the guide wire may be removed or left in place to guide passage of a choledochoscope or baskets. When the choledochoscope is used, a second incision in the cystic duct, close to the CBD, avoids Heister's valves and allows removal of the guide wire. If baskets are used, 6 French plastic introducer sheath may be inserted through the trocar used for cholangiography into the cystic duct. This sheath is especially useful if multiple stones must be removed.
- Laparoscopic CBD exploration:
- Large stones (> 1 cm), as well as most stones in the common hepatic ducts, are not retrievable with the techniques described above. Ductal clearance can be achieved via choledochotomy if the duct is dilated and the surgeon is sufficiently experienced. (46, 47)
- The anterior wall of the CBD is bluntly dissected for a distance of 1 to 2 cm. When small vessels are encountered, it is preferable to apply pressure and wait for hemostasis rather than use the electrocautery in this area. Adrenaline-soaked gauzes placed' through the 12 mm umbilical port are very effective for this purpose. Two stay sutures are placed in the CBD.
- An additional 5 mm trocar is placed in the right lower quadrant for insertion of an additional needle driver. A small longitudinal choledochotomy (a few millimeters longer than the circumference of the largest stone) is made with curved microscissors on the anterior aspect of the duct while the stay sutures are elevated.
- A choledochoscope is then inserted and warm saline irrigation initiated. In most cases, baskets should suffice for stone retrieval; however, lithotriptor probes and lasers are available for use through the working channel of the choledochoscope. The choice of approach depends on' availability and individual surgical experience.
- Subsequently, a 12 or 14 French latex T tube is fashioned with short limbs, placed entirely intraperitoneally to prevent C02 from escaping, and positioned in the CBD. The choledochotomy is then closed with fine interrupted absorbable sutures
- The first suture is placed right next to the T tube, securing it distally, and the second is placed at the most proximal end of the choledochotomy; lifting these two sutures facilitates placement of additional sutures. Intracorporeal knots are preferred to avoid sawing of the delicate tissues. The end of the T tube is then pulled out through a trocar, and cholangiography is performed after completion of the procedure.
- Stones in the common bile duct
- Laparoscopic common bile duct exploration
- Open Choledocholihotomy:
The same procedure clan be done, with open method when surgeon is uncomfortable doing laparoscopic CBD exploration.
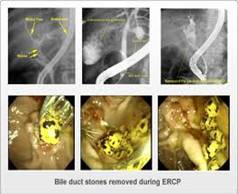
- Endoscopic retrograde cholangiopancreatography (ERCP):
- Endoscopic retrograde cholangiopancreatography (ERCP) is technique that combines the use of endoscopy and fluoroscopy to diagnose and treat certain problems of the biliary or pancreatic ductal systems. Through the endoscope, the physician can see the inside of the stomach and duodenum, and inject dyes into the ducts in the biliary tree and pancreas so they can be seen on x-rays.
- ERCP is used primarily to diagnose and treat conditions of the bile ducts, including gallstones, inflammatory strictures (scars), leaks (from trauma and surgery), and cancer. ERCP can be performed for diagnostic and therapeutic reasons, although the development of safer and relatively non-invasive investigations such as magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound has meant that ERCP is now rarely performed without therapeutic intent.
Complications:
1. Anaesthesia related problems:
Among the potential complications of general anesthetics are hypoventilation, esophageal intubations; gastroesophageal reflux, bronchospasm, hypotension, narcotic overdose, cardiac arrhythmias and cardiac arrest. CO as an insufflation, medium causes a decrease in parameters like p02, 02 saturation, tidal volume and minute ventilation and an increase in respiratory rate. Elevation of diaphragm causes basilar atelectasis, right to left shunt and hence ventilation per-fusion mismatches and decreased respiratory compliance.
2. Pneumoperitoneum related problems:
- Carbon dioxide embolism:
- C02 is the most widely used peritoneal distension medium. Most C02 micro emboli are absorbed, usually by the splanchnic vascular system, quickly and without incident. However sever cardio respiratory compromise may result if large amount of C02 gains access to the central venous circulation, such as with inadvertent intravascular placement of an insufflation needle.
- Diagnosis confirmed by sudden, unexplained hypotension, cardiac arrhythmia cyanosis, development of a classic 'mill wheel murmur', increased tidal C02, findings of pulmonary edema and pulmonary hypertension resulting in right side heart failure.
- Treatment includes immediate intraperitoneal gas evacuation, 100°;0 02 administration, vasopressor support and hyperventilation to aid elimination of C02. Durant's position (left lateral position) displaces gas from right ventricular outflow pulmonary tract to right ventricle, thus relieving the functional obstruction.
- Cardiovascular complications:
Cardiac arrhythmias can develop commonly due to hypercarbia and resulting acidemia.
Operating at pressures less than 12 mm of Hg can prevent these. Pneumothorax is also a known complication of positive pressure ventilation due to high peak airway pressure.
- Gastric reflux:
Gastric regurgitation and aspiration are problems increased due to Trendelenburgh position and also increased intra-abdominal pressure These can be avoided by fasting at least 8 hours prior to surgery and for introduction of a nasogastric tube to empty the stomach.
3. Veress needle and trocar injuries.
GI. Tract injury:
- Needle entry into abdominal GI. tract organs may occur and commonly is in the stomach due to gastric distention or when adhesion binds the stomach to the abdominal wall. The injury is often created by the primary trocar because of its blind insertion.
- Recognizing gastric entry by the insufflation needle may follow identification of signs of extraperitoneal entry, such as increased filling pressure, asymmetric distention of the abdomen, or the aspiration of gastric particulate through the lumen of the needle. Colonic injury is manifested by feculent odour. Such injuries are Common in patients with previous surgeries where the bowel is adherent to the anterior abdominal wall. Risk of injury can be significantly reduced by liberal use of oral or nasogastric decompression. Consequently, preoperative mechanical bowel preparation should be used in high-risk cases.
- Also use of left upper quadrant for insertion of the first trocar can be used. This approach allows direct insulation of abdominal wall under the umbilicus or other planned site of insertion and may facilitate dissection of underlying adhesion.
- The management depends on the nature of injury. Insufflation needle punctures that have not caused a defect significantly larger than their diameter can be handled expectantly. Large defects should be repaired by laparoscopic or laparotomy based approach. Extensive lesions may require resection and reanastomosis
- Urologic injury.
- Injury to the bladder may occur during insertion of Veress needle or trocar. These are more often when bladder is adherent to the anterior abdominal wall after pelvic surgery. The diagnosis is easy if the surgeon recognizes entry into the hollow viscous or urine in the operative field. Hematuria suggests urinary tract injury and pneumaturia is diagnostic of visceral entry. The existence of bladder injury can be confirmed by instilling methelene blue in an indwelling catheter. The injury can be easily prevented by routine preoperative bladder drainage and thorough knowledge of anatomy.
- Small caliber (l-2mm) injuries heal spontaneously by prolonged catheterization (1 -2wks). Significant injury (above 2cm) can be repaired laparoscopically or open technique by either single or double layer with absorbable sutures. For injuries involving the trigone, open repair is preferred. Similarly ureteric injuries can be treated by insertion of a ureteric stem kept for I5-20 days.
c) Vessel injury.
Great vessel injury:
- Injury to the great vessel like aorta, common iliac vessels, internal and external iliac vessels can occur during insertion of veress needle or trocar. Often the manifestation is egress of blood through the cannula and sudden hypotension. Frequently bleeding is contained in the retroperitoneal space, which delays diagnosis. The management consists of immediate laparotomy and arrangement of blood for transfusion.
Abdominal wall vessel injury;
- Commonest vessel injured is the superficial inferior epigastric vessels as they branch from the femoral artery and course cephalad, The problem is recognized by observation of blood dripping along the cannula or out through the incision. These injuries can be managed by application of pressure by change of position of cannula, pressure by Foleys catheter balloon hitched to the abdominal wall or placing a horizontal mattress suture to be removed after 48hrs.
4) Thermal injury:
- Thermal injury is usually to the bowel due to inadvertent use of electrocautery. The injuries are caused due to current diversion and active electrode. The injury is recognized, estimating the extent of the damage usually is difficult because the zone of dissection may exceed the area of visual damage.
- These thermal injuries can be prevented by adequate exposure of the operative field, performing the dissection near vital structures by scissors, clipping of blood vessels near the bowel rather than cauterizing them and judicious use of electrocautery by using short spurts of current.
- Principles of electrosurgical safety to be followed included use of electro surgical of acceptable standards, keeping instruments in good working condition with intact insulation, avoiding intra and extraperitoneal injuries from inadvertent activation, adequate exposure by panoramic view and using the ideal waveform and power output with appropriate active electrodes and technique.
5) Early and late post operative complications:
- It commonly results from preoperational placement of an insufflation needle or leakage ofC02 around the cannula sites, the latter frequently because of excessive intraperitoneal pressure.
- The emphysema can be readily identified by palpation of crepitus on the abdominal wall. If it extends into the mediastinum, it may lead to pneumothorax and cardio vascular collapse.
- Preventive measures include confirmation of intraperitoneal placement of needle, initiating insufflation at a low rate (1 lit/mill), confirming symmetrical abdominal distention and obliteration of liver dullness and operating at low intraperitoneal pressure after placement of all cannulas.
- In mild cases, no specific intra or postoperative therapy is required. But in severe cases the procedure is preferably terminated.
- Incisional Hernia and wound dehiscence.
- It is commonly seen in defects that are 10 mm or larger in size. Cases of bowel herniation and intestinal obstruction have been reported. These are mainly caused when the rectus sheath is not closed properly.
- Preventive measures include the use of smallest size cannula necessary for completing the procedure and adequate approximation of the rectus sheath under vision to ensure that bowel is not trapped inside. Even laterally placed 10 mm ports should be closed.
c) Intra-abdominal abscess.
- Commonly seen after appendectomy more in infected and gangrenous cases. To avoid this, one must be careful in removing the appendix from the abdomen, taking care not to contaminate other tissues, and ensure that no fecal contamination or appendiceal fragmentation occurs during appendectomy. Abdominal irrigation is helpful in preventing the development of pelvic sepsis. Broad-spectrum antibiotics are indicated.
d) Ileus
May be due to excessive bowel manipulation or intraoperative contamination. Treatment includes nil by mouth, Nasogastnc aspiration and correction of hypokalemia, hypoproteinemia and early mobilization of the patient.
e) Wound infection
f) Complications specific to cholecystectomy: |
Common bile duct injuries.
- Studies estimated the rate of bile duct injury during an open cholecystectomy to be from 0.053% to 0.6%,but the accepted standard is a rate of 0.1 % to 0.2%.(20,21,22,23) Laparo- scopic cholecystectomy, in contrast, is associated with a bile duct injury rate in the range of 0.2% to 2%.
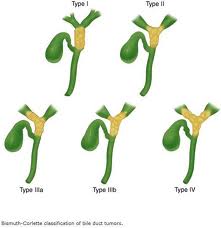
- Injury is broadly defined and includes everything from cystic bile-duct leaks to proximal segmental hepatic duct ligation and excision. Bismuth classified bile duct strictures on the basis of their location with respect to the hepatic duct confluence. However, biliary leaks (choledochal, hepatocystic, or sectoral) and isolated sectoral duct occlusions are not included in this classification scheme, and these account for a large proportion of the injuries following laparoscopic cholecystectomy.
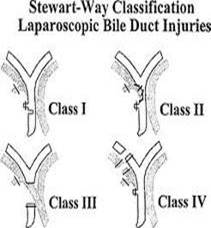
- Strasberg's classification scheme, proposed in 1995, reflects the diversity of biliary injuries and their management(27). This scheme includes Bismuth's classification of strictures as its highest injury category.
Presentation, Mechanism and Management
- Strasberg type A injuries include leaks from the cystic duct stump or a minor duct on the liver bed, but biliary-enteric continuity is intact. Leakage from the cystic duct occurs secondary to clip failure or a burst phenomenon in the presence of retained common duct stones; it may also be seen when the cystic duct stump necroses, strangulated by clips that are simply too tight. (Minor duct leaks are usually caused by separating the gallbladder from the gallbladder fossa in a plane that is too deep, that is, excising a rim of liver with the specimen, thus injuring a subvesical duct or a hepatocystic duct of Luschka(26)
- Patients with this-type of injury present with symptoms related to the presence of intraperitoneal bile: abdominal pain, anorexia, ileus, nausea, or bile peritonitis with sepsis; the latter presentation may mandate laparotomy.
- These leaks are often treated with endoscopic trans ampullary stenting or sphincterotomy to decrease endo biliary pressure and percutaneous drainage of localized bile collections; It should be stressed that postoperative fluid collections are very common but rarely clinically significant, Almost 10% of patients who undergo laparoscopic cholecystectomy are found postoperatively to have intraperitoneal bile,(27) yet,, less than 1 % of all laparoscopic cholecystectomies are complicated by a clinically detectable leak.
- Type B injuries occlude a portion of the biliary tree and may occur when the cystic duct drains into the right hepatic duct rather than the common duct as is seen in small segment of the population
- Patients with type B injuries may remain asymptomatic as the obstructed lobe or segment atrophies and the remaining liver hypertrophies; alternatively, they may present with pain or cholangitis in the occluded area.
- If the patient is symptomatic, management includes completely defining the anatomy with percutaneous and endoscopic cholangiograms, placement of drains for the control of sepsis, and subsequent Roux -en- Y hepaticojejunostomy by experienced operators Timing of operation depends upon when the injury was recognized. An injury recognized intraoperatively should be repaired immediately if the expert resources are available; if appropriate hepatobiliary expertise is not immediately available, a drain should be secured in the proximal limb of the ligated duct, the patient should be closed, and transfer to a tertiary care facility arranged. Injuries recognized postoperatively should be repaired only after accompanying sepsis has resolved.
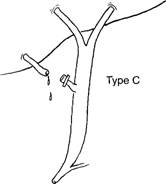
- Type C injury is a leak from a duct not in continuity with the common duct, that is, a sectorial duct injury.
- Definitive management includes Roux-en-y hepaticojejunostomy if the leaking duct is large (i.e., draining two or more segments), or (ligation if the duct is small).
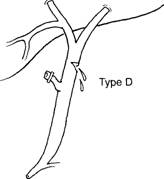
- Type D injury is a lateral injury (not a complete transection) to any extrahepatic duct, and, like many 'injuries, may be caused by cautery, scissors, or improper placement of clips.
- The severity and location of the injury determine its presentation, clip or scissor injury to the right hepatic duct, for example, might present as a leak with biliary-enteric continuity intact, much like a type A injury.
- Stenting across the injury or distally may be helpful, but if a stricture develops, repair may be necessary, common duct leak may be repaired over a 'l-tube, in end-to-end fashion, or by Roux-en- Y hepaticojejunostomy, but the end-to-end repair is commonly plagued by tension on the anastomosis.
- type D injury may be asymptomatic, as when a cautery injury to the common duct does not cause a leak; a patient with such an injury may present years postoperatively with a stricture, with or without jaundice, cholangitis, or liver failure.
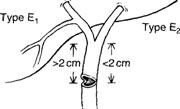
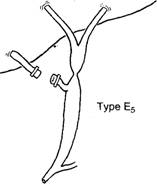
- Type E injury is an excision or complete occlusion of the common hepatic or common bile duct that totally disrupts biliary-enteric communication. It usually occurs when there is a severe cautery injury to a major duct or its blood supply; or when the cystic duct is ligated too close to the common duct.
- Type E injuries most commonly present with jaundice, recurrent cholangitis ,pain, and sepsis. Presentation may be subtle, as only 10% of post cholecystectomy strictures are detected within a week of the initial operation. 80% are detected within the first year, a stricture may not become evident for several years following the injury; this not only complicates efforts to determine the incidence of such injuries, but, more important, puts the patient at risk for cirrhosis and portal hypertension.(28,29)
- The management goal in treating a type E injury is to reestablish biliary-enteric continuity in a fashion that minimizes the chance of restricture with subsequent cholangitis and liver failure Successful management of the type E injury must include (1) completely defining the nature of the injury with cholangiography from above and below, (2) percutaneously draining the entire biliary system. (3) treating sepsis and periportal inflammation with antibiotics and drainage of collections. and (4) appropriately timing an expert, definitive repair) The focus should be on preventing the need for future re-repair, It should be emphasized that once sepsis is con- trolled and the biliary system is drained, there is no urgency to perform the definitive repair. Periportal inflammation can make it nearly impossible to expose healthy ductal mucosa suitable for anastomosis, so immediate repair of an injury discovered postoperatively may not be advisable.
  
  
Strasberg classification
The first repair has the greatest chance of success.
- Bismuth classification
- Stewart-way classification
Hepaticojejunostomy:
- Injury to common bile duct during surgery warrants a surgeon to do a reconstructive procedure. Hepaticojejunostomy is the procedure of choice for the CBD injury. Although different remedies have been ascribed for repair of CBD, but Hepaticojejunostomy still remains the gold standard procedure.
- The principle of the procedure is that a roux limb is created at a distance of about 40-50 cms. From the ligament of Treitz. Depending upon the Bismuth grade of injury to the CBD, the proximal part of CBD is so fashioned, that a end to side Hepaticojejunal anastomosis is done. The elementary limb of the jejunum is anastomosed end to side with roux limb of the jejunum at a distance of about 10-20 cms, from the Hepaticojejunal.
Recent advances
Single port laparoscopic cholecystectomy
- A new operating technique is gaining ground, which might be considered as a further evolution of Minimally Invasive, or Minimal Access Surgery. It is called by different names, but the principle remains the same:
- To further reduce the access trauma and the visibility of scars, by placing the access centrally and exclusively in the umbilicus. More specifically one might further distinguish single port and single site surgery. Whereas single port surgery - as indicated by its name - uses only a single, but relatively big device for access, single site surgery involves up to four trocars, but still exclusively placed through the umbilicus.
- Quite a few names have been coined in the meantime about it as SPA (Single Port Access), Single Access Surgery (SAS), E-NOTES (Embryonic-NOTES) or by similar terms, or S-PORTAL, or SILS (single incision laparoscopic surgery)
- One major difficulty in S-PORTAL procedures is the reduction of free maneuvering space and the potential collision of instruments and telescope.
 
Benefits of single incision laparoscopic surgery:
Transumbilical approach provides the potential for no visible scar
Surgical incision may be hidden in the umbilicus
Minimizes the scars that may accompany additional sites of. entry
Potential for less post-operative pain due to the elimination of 3 additional puncture sites
Reticulating Instruments Provide Optimal Access and Ease of Use
0- to 80-degree articulation
Freedom of tip placement
360-degree rotation at all articulation angles
Spin-lock rotation position lock
Perform as a fully rigid straight instrument
Left- or right-hand versatility
Optimal access to difficult anatomy and maximum ease of use
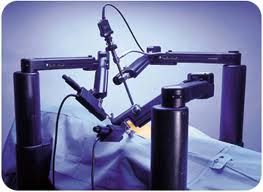
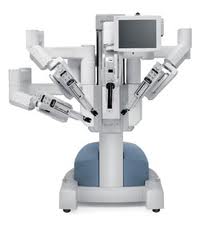
Robotic surgery
- Robotic surgery, computer-assisted surgery, and robot-assisted surgery are terms for various technological developments that currently are developed to support a range of surgical procedures.
- Robot-assisted surgery was developed to overcome limitations of minimally invasive surgery, Instead of directly moving the instruments the surgeon uses a computer console to manipulate the instruments attached to multiple robot arms. The computer translates the surgeon's movements, which are then carried out on the patient by the robot. Other features of the robotic system include, for example, an integrated tremor filter and the ability for scaling of movements (changing of the ratio between the extent of movements at the master console to the internal movements of the instruments attached to the robot). The console is located in the same operating room as the patient, but is physically separated from the operative workspace. Since the surgeon does not need to be in the immediate location of the patient while the operation is being performed, it can be possible for specialists to perform remote surgery on patients. Robots can perform surgery without a human surgeon
Advantages and disadvantages
- Major advances aided by surgical robots have been remote surgery, minimally invasive surgery and unmanned surgery. Some major advantages of robotic surgery are precision, miniaturization, smaller incisions, decreased blood loss, less pain, and quicker healing time. Further advantages are articulation beyond normal manipulation and three-dimensional magnification, resulting in improved ergonomics. Robotic techniques are also associated with reduced duration of hospital stays, blood loss, transfusions, and use of pain medication.
- With a the cost of the robot at $1,200,000 dollars and disposable supply costs of $1,500 per procedure, the cost of the procedure is higher. Additional surgical training is needed to operate the system. Patient surveys indicate they chose the procedure based on expectations of decreased morbidity, improved outcomes, reduced blood loss and less pain. Higher expectations may explain higher rates of dissatisfaction and regret.
- The main advantage of this technique is that the incisions are very small and, consequently, patient recovery is quick. In traditional open-heart surgery, the surgeon makes a ten to twelve-inch incision, then accesses the heart by splitting the sternum (breast bone) and spreading open the rib cage. The patient is then placed on a heart-lung machine and the heart is stopped for the length of the surgery. Not only is this a way for bacteria that can cause infections to access the patient's body, it also leads to a painful wound, which takes time to heal.
- Because patient recovery after robot-assisted heart surgery is quicker, the hospital stay is shorter. On average patients leave the hospital two to five days earlier than patients who have undergone traditional open-heart surgery and return to work and normal activity 50%) more quickly. Reduced recovery times are not only better for the patient, they also reduce the number of staff needed during surgery, nursing care required after surgery, and, therefore, the overall cost of hospital stays.
In 2007, the. University of Illinois at Chicago medical team, lead by Prof. Pier Cristoforo Giulianotti, performed the world's first ever robotic pancreatectomy and also the Midwests fully robotic Whipple surgery. In April 2008, the same team of surgeons performed the world's first fully minimally invasive liver resection for living donor transplantation, removing 60% of the patient's liver, yet allowing him to leave the hospital just a couple of days after the procedure, in very good condition. Furthermore the patient can also leave with less pain than a usual surgery due to the four puncture holes and not a scar by a surgeon
Conclusion.
Thus laparoscopic cholecystectomy has become the gold standard for treatement of gall stone disease. Knowledge of anatomy and anatomical varients goes a long way in avoiding complications during surgery and also management of post operative status.Robotics and single incision surgery , due to obvious benefits are here to stay and may soon become gold standards with decreasing costs and instrument innovations.
|
|